Contract Services
Changing the shape of GMP peptide manufacture 2nd September 2019
By Alastair Hay, PhD, Account Manager – Peptides, Almac
Alastair Hay, PhD, Account Manager – Peptides at Almac, explains how the company is advancing human health through innovation an
Alastair Hay, PhD, Account Manager – Peptides at Almac, explains how the company is advancing human health through innovation and investment in the field of individualized cancer vaccines.
Background
At Almac we first became aware of the field of individualized cancer vaccines when we were asked, “Do you think it would be possible to make 20mg of 20 peptides under GMP in less than 4 weeks?” At that time, Almac had spent a few years establishing its GMP peptide business, but this was very different from anything we’d been asked previously. We knew we were good at making non-GMP peptides quickly, through more than 20 years of operating our custom synthesis business, so we wondered if we could combine the two aspects of high speed manufacture and GMP compliance.
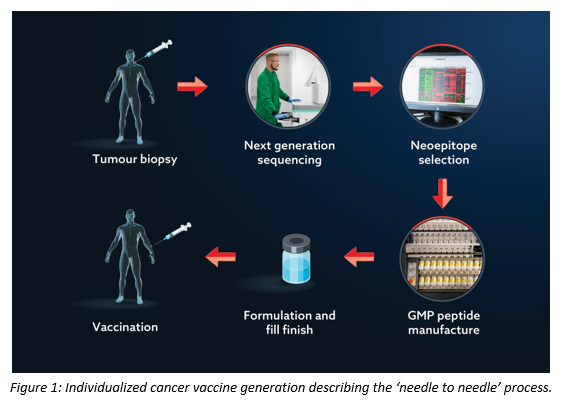
Before that initial enquiry we were not aware of any neoantigen-based therapeutic strategy. We knew of tumour-associated antigen-based cancer vaccines, but to that point they had not fulfilled their promise, so it was interesting to understand what might be different about this new individualized approach. The aim of the neoantigen-based strategy is to get round the issues faced by traditional cancer vaccines (which are too much like ‘self’ for the immune system to see them as foreign and hence be stimulated into destroying cancer cells) by individualizing the treatment (Figure 1). In brief, a tumour biopsy undergoes next generation sequencing (NGS) to identify the mutations present. A variety of techniques are then used to determine which mutations might result in an antigen that, in turn, could elicit an immune response. Those ‘neoantigens’ are used as the basis of the vaccine design. The peptides replicate the neoantigens; they are manufactured to GMP, formulated, and the patient inoculated, often in combination with other treatment forms, such as a checkpoint inhibitor.
Clearly, for the patient’s benefit, the entire process must be carried out as quickly as possible. Whilst peptide manufacturing is only one link in the chain, it is the most complex manufacturing operation and one that is perceived as a major hurdle to overcome.
Manufacturing set-up
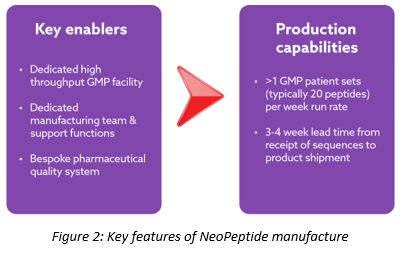
So how do you go about manufacturing 20 GMP peptides in less than 4 weeks? That took us at Almac a little while to work out. To do so, we stripped things back to first principles. We spent a long time mapping the manufacturing process and flow, understanding where the bottlenecks were, and identifying what equipment, procedures and head count we’d need (Figure 2).
Key to our success was getting to grips with the GMP and regulatory requirements. There is no precedent for the neoantigen field in a regulatory setting, and so we have had to set expectations, gaining feedback from the authorities at every step. Almac does not subscribe to a “GMP-light” philosophy – the manufacturing approach for us had to be a fully compliant GMP approach.
Early on in designing our set up we consulted with the MHRA (UK authorities) on how we planned to implement GMP; we walked them through our process and facility set-up and described our proposed procedures. In parallel, we supported clients through their submissions to various authorities around the globe. This required a great deal of client intimacy as each had a slightly different set of regulatory requirements to adhere to. The outcome for Almac is a GMP-certified facility capable of producing 20 GMP peptides in less than 4 weeks. This new NeoPeptide facility, based in Edinburgh, came online with a single GMP line in September 2018. Very quickly we introduced a second GMP manufacturing line. With two lines in operation we are able to produce peptides at a rate of more than 2 patients per week (more than 40 GMP peptides per week). The duration of manufacture for each patient is typically 3-4 weeks, and manufacture for multiple patients occurs concurrently within our facility which is modular and segregated to strictly prevent cross-patient contamination.
Future outlook
In June 2019, we invested in a 4-fold expansion of our facility, which we describe as our ‘one patient per day suite’. This bespoke facility includes state of the art manufacturing and analytical equipment, and dedicated manufacturing lines to ensure patient segregation. The new facility is designed to produce at a rate of one patient per day (i.e. 20 GMP peptides per day), with each patient manufacturing duration being 7 days (from receipt of order to peptide shipment). This is equivalent to a manufacturing speed which is 1000x faster than that of conventional peptides. Achieving such a manufacturing rate is made possible through a combination of process intensification, technological development, highly refined facility design and appropriate staffing levels with highly trained and exceptionally dedicated staff.
As more clinical trials come to the fore, the regulatory landscape is likely to evolve. There are currently very few guidelines, but this is likely to change as experience grows in such a fledgling field. The fact that dosage is so low and products are individualized clearly means that the population risk profile is very different to that of conventional therapeutics, which may present an exciting opportunity to create more unique solutions for clients that satisfy regulatory questions in future.
Author:
Alastair Hay is Account Manager – Peptides at Almac Sciences, Technophone, Milton Bridge, Tranent EH26 0BE, UK
+44 (0) 28 3839 5717
NeoPeptide is a registered trademark of Almac Group.



