Contract Services
CMOs offering real-time data analytics sharing are ahead of the curve 19th July 2019
By Peter Guilfoyle, Vice President – Marketing at Northwest Analytics
Peter Guilfoyle, Vice President – Marketing at Northwest Analytics, explains how digitalization is enabling pharmaceutical compa
Peter Guilfoyle, Vice President – Marketing at Northwest Analytics, explains how digitalization is enabling pharmaceutical companies and CMOs to use shared analytics platforms that enable access to real-time information about the quality and quantity of products under manufacture.
Digitalization is a global mega-trend sweeping across almost every industry. Core to any digitalization program are the source data and analytics – what’s required to transform huge quantities of raw data into usable, actionable and valuable information. Within the pharmaceutical industry, manufacturing analytics is already being applied to produce drugs more consistently, more quickly and with improved process safety.
At the same time, manufacturing analytics are making it easier to adhere to regulatory requirements with – for example – real-time generation of Continued Process Verification (CPV) and batch reports. In fact, leveraging manufacturing analytics for CPV Stage 3 delivers results at all turns:
- Enabling companies to see and proactively address process drifts
- Improving production performance
- Easing the burden and cost of regulatory compliance
- Understanding exactly how API and excipient suppliers might impact product quality
- Minimizing the potential risk associated with outsourced production.
When a pharmaceutical company outsources the production of its drugs to a contract manufacturing organization (CMO), a huge amount of trust involved. How can they be sure that the CMO will execute as needed? How do they know under what conditions product is made? How do they know corrections to processes are made before final product quality is impacted? How do they know what’s delivered is not just meeting specifications but, more importantly, also fit-for-use and compliant with regulatory requirements and guidelines?
Taking a page from some of their key suppliers, pharmaceutical companies can address these needs directly with manufacturing analytics platforms that enable them to access real-time analysed data (not a CMO’s raw data) relating to the conditions and control of processes related to their products. This digital view of relevant process analytics enables a manufacturing transparency that engenders trust, collaboration and confidence while minimizing the potential risk associated with outsourced production.
The approach also benefits the CMO, allowing the distribution of relevant information (not raw data) to the customer without them demanding to ‘dig around’ in the raw process data. The CMO can also apply the platform upstream to its own suppliers, to ensure the quality and consistency of ingredients. These benefits, combined with the general returns of manufacturing analytics – making the highest quality pharmaceuticals at the highest speeds and volumes, with minimal waste and meeting regulatory requirements – offer considerable competitive advantages when considering a CMO’s service offerings alongside those of others.
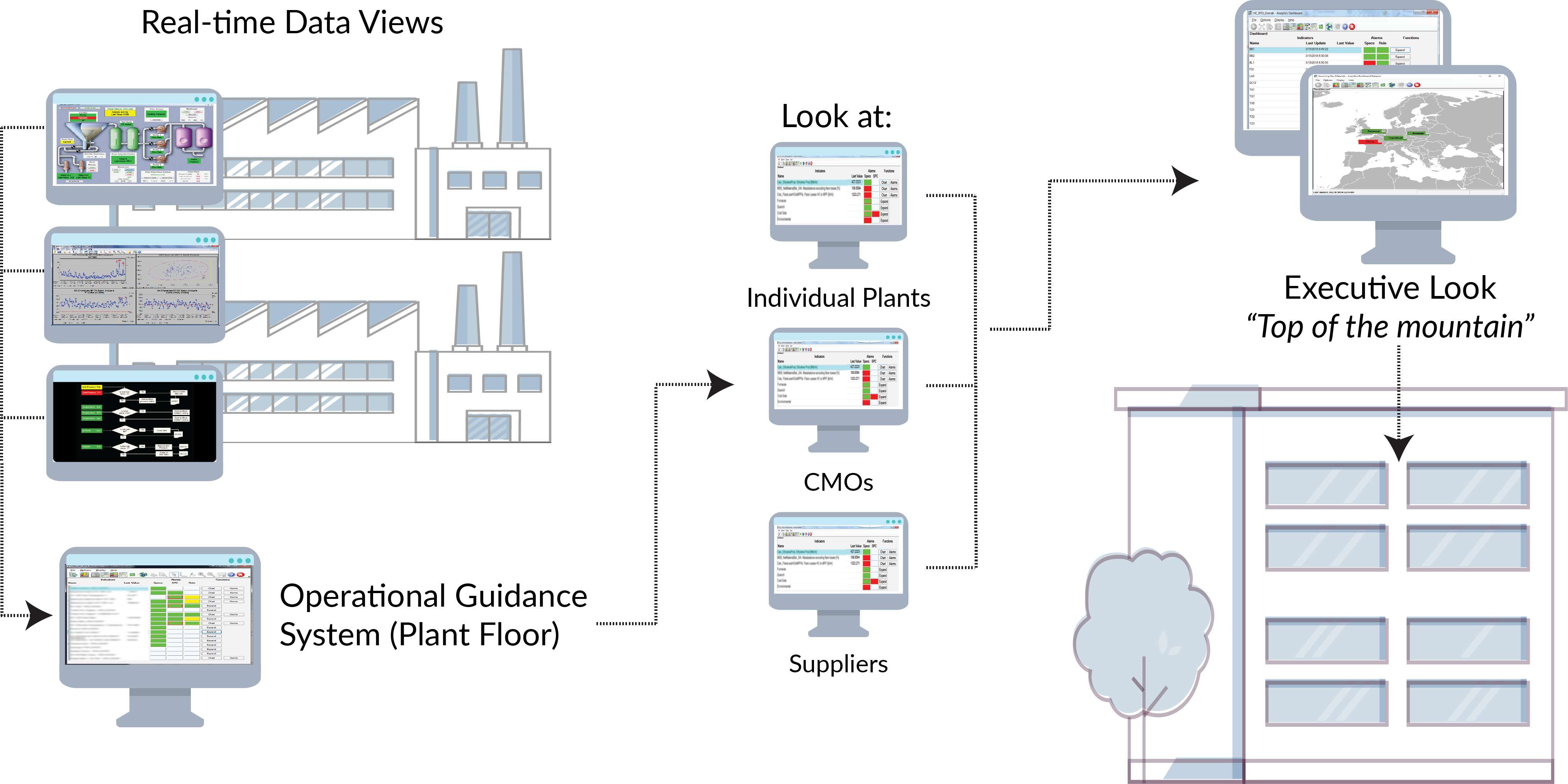
Such platforms can be implemented using technologies that are already validated and in place, enabling the results to be presented, in context, to a variety of users throughout the CMO-customer chain. Using bespoke dashboards for each type of user – from operators to plant managers at the CMO to the procurement team or manufacturing leaders at the pharmaceutical company – ensures that every person consuming the analytics can easily see the information that is important to them in a straightforward and actionable way (see Figure).
This construct means that the very data being used to run a plant – or a CMO – also enables a higher-level view of production. Whether production is in-house or at a CMO, understanding who is doing well and who is challenged is key for managing both supply and potential risk. In a recent case, a pharmaceutical customer used this bottom’s up view to provide comparisons of its internal and CMO plants, closely monitoring each with an analytics view of key performance metrics as well as a measure that indicated each facility’s overall CPV Stage 3 performance.
It seems inevitable that it will soon be standard for CMOs to provide this analytics-centric view of processes and products to their customers – or for pharmaceutical companies to demand that of their supply chain. This manufacturing analytics-based approach reduces the risk for everyone involved.
Access to real-time information is already the norm. Our lives are infused with real-time digital experiences, and it’s only natural to expect the same level of connectivity and transparency from our suppliers at work. CMOs not already embarking on their digital transformation need to pick up the pace or they risk not just being left behind, but being left out of the game altogether.
Contact:
Tara Sybrant, Marketing Director at Northwest Analytics Inc, 111 SW Fifth Avenue, Suite 800, Portland, OR 97204-3606, USA
T: +1 503 224 7727
E: tsybrant@nwasoft.com



