Contract Services
Developing an industrial process for manufacturing hydromorphone hydrochloride through DoE and optimization 6th June 2018
By Par Holmberg, Senior Scientist, Cambrex
Par Holmberg, Senior Scientist at Cambrex, describes a case study applying process optimization to reduce the number of synthetic
Par Holmberg, Senior Scientist at Cambrex, describes a case study applying process optimization to reduce the number of synthetic steps, eliminate hazardous reagents and allow for easier material handling of an existing, mature product, affording a new, efficient process to ensure longevity of the product in the future.
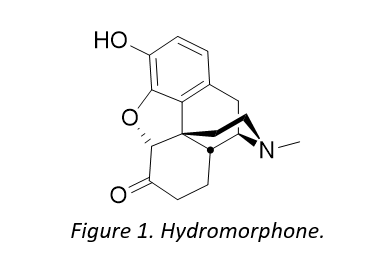
Cambrex already had in its technological portfolio a highly efficient process for making hydrocodone via a redox isomerization of concentrate of poppy straw (CPS) rich in codeine and when looking for a new synthetic route to hydromorphone, the first thought was to perform a 3-O-demethylation of hydrocodone.
3-O-demethylations of opiates can either be performed under acidic or basic conditions, however, hydromorphone is intrinsically unstable under basic conditions, so that option was ruled out.
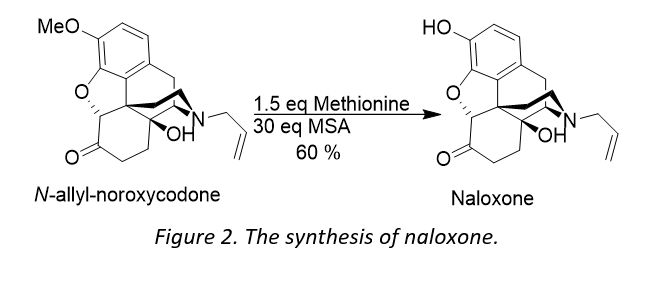 A survey of the relevant literature for 3-O-demethylations of opiates provided an interesting paper published in 1992, by Andre et al.1 The authors discuss the use of a methane sulfonic acid (MSA) / methionine acting as a hard acid / soft nucleophile system in the 3-O-demethylation of different opiates, a method originally reported by Yajima2 and Kiso3 in the deprotection of peptides. The paper reported how naloxone, which is structurally similar to hydromorphone, can be made from N-allyl noroxycodone. Naloxone exerts an antagonistic effect on the opioid receptor and has recently received wide attention from the media in its use of treating opioid overdoses.
A survey of the relevant literature for 3-O-demethylations of opiates provided an interesting paper published in 1992, by Andre et al.1 The authors discuss the use of a methane sulfonic acid (MSA) / methionine acting as a hard acid / soft nucleophile system in the 3-O-demethylation of different opiates, a method originally reported by Yajima2 and Kiso3 in the deprotection of peptides. The paper reported how naloxone, which is structurally similar to hydromorphone, can be made from N-allyl noroxycodone. Naloxone exerts an antagonistic effect on the opioid receptor and has recently received wide attention from the media in its use of treating opioid overdoses.
Andre et al treated N-allyl-noroxycodone with 30 equivalents of MSA and 1.5 equivalents of methionine (Figure 2) for 15 hours at 40°C, affording naloxone in 60% yield after recrystallization.
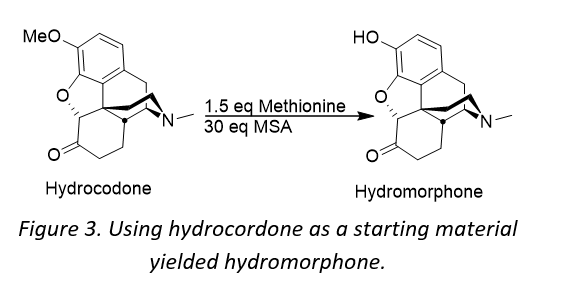
It became clear that the appropriate choice of the hard acid and soft nucleophile was essential for the success of developing a manufacturing process for hydromorphone. What followed was a development process involving screening various combinations of acid, co-acid and sulfide. The use of a DynaBloc heater, combined with Design of Experiment (DoE) software afforded the generation the qualitative/quantitative results within a short time frame. The DynaBloc heater allows 18 reactions to be run in parallel, and DoE is a systematic method to determine the relationship between factors affecting a process and the output of that process. The DoE software enabled reaction model prediction by statistical analysis, allowing the team to better assess where to direct their process development. In all, over 450 reactions were run using a combination of 6 acids, 11 co-acids and 40 different sulfides.
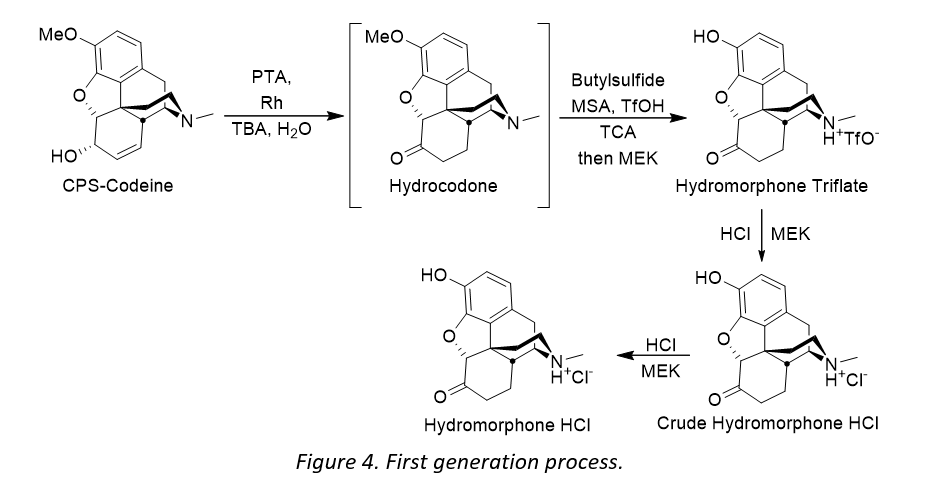
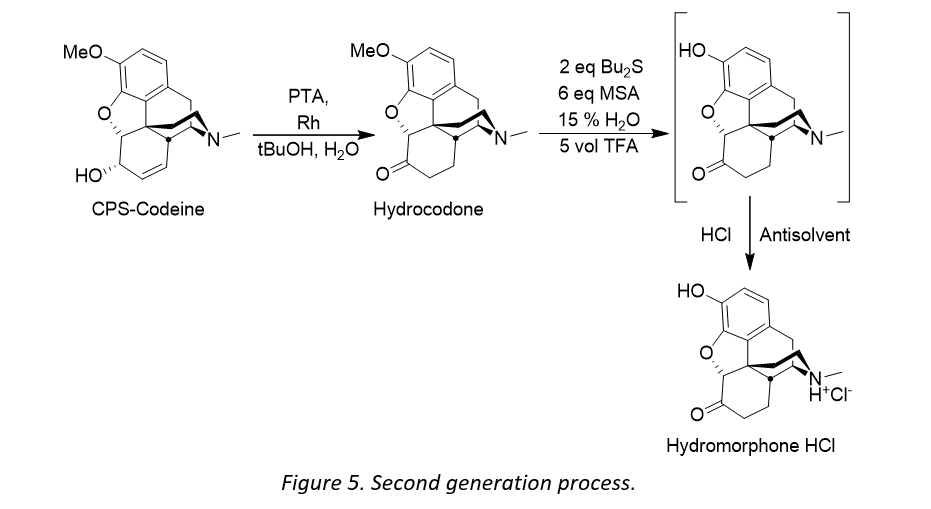
- Remove the use of triflic acid to eliminate materials of construction and safety issues
- Switch the hygroscopic solid trichloracetic acid to trifluoroacetic acid (TFA) to improve handling
- Remove one step in the process by eliminating the salt swap i.e. isolating hydromorphone hydrochloride directly from the reaction mixture.



