Contract Services
Who are the key players in the CDMO industry? 10th October 2018
By our Editorial Team
The global contract development and manufacturing organization (CDMO) market is flourishing, but it can be difficult to obtain cle
The global contract development and manufacturing organization (CDMO) market is flourishing, but it can be difficult to obtain clear data that facilitate industry decisions regarding outsourcing, or investments in this sector. Advitech has addressed this challenge with an extensive benchmarking study of CDMOs working in the small molecule API space.
The rise of CDMOs is a key aspect of the pharmaceutical industry’s evolution over the past decade. The quote, “Do what you do best and outsource the rest” (attributed to Peter Ferdinand Drucker, 1909–2005), is a perfect description of today’s approach, which sees outsourcing as a consolidated aspect of the pharmaceutical industry. The global CDMO market is currently experiencing a high single-digit growth rate, with a 7.5% compound annual growth rate (CAGR) forecast from 2015-2019.1 The success of the CDMO sector is reflected in a recent report that two-thirds of pharmaceutical manufacturing is outsourced.2
However, despite the wealth of information publicly available via the internet and other sources, it can be difficult to extrapolate the data required to make informed decisions about outsourced pharma manufacturing, or investments in this sector. To address this challenge, the Swiss company Advitech Advisory and Technologies recently performed its first benchmarking study on APIs Trends: A CDMO Benchmarking Report, with a specific focus on companies producing small molecule APIs.
What’s it worth?
The study started with an evaluation of the market value of drug substances produced by CDMOs. While the market value of finished dosage forms (FDFs) manufactured by CDMOs can be relatively easily assessed, values for APIs are not as visible. Therefore, to obtain an estimated value of the market pertaining to the subsequent benchmarking study, Advitech performed a series of intelligent extrapolations from a range of publicly available and published data. The analysis provides rare insight, placing an actual figure on the global market for drug substance APIs.
Who’s doing it?
Advitech’s benchmarking study began with the selection of 27 companies, which were chosen due to their well-established role as contract manufacturers and small molecule API capabilities. The companies chosen for the study were mainly headquartered in Europe or in the US, although two best-in-class companies in Asia were also included to provide additional perspective.
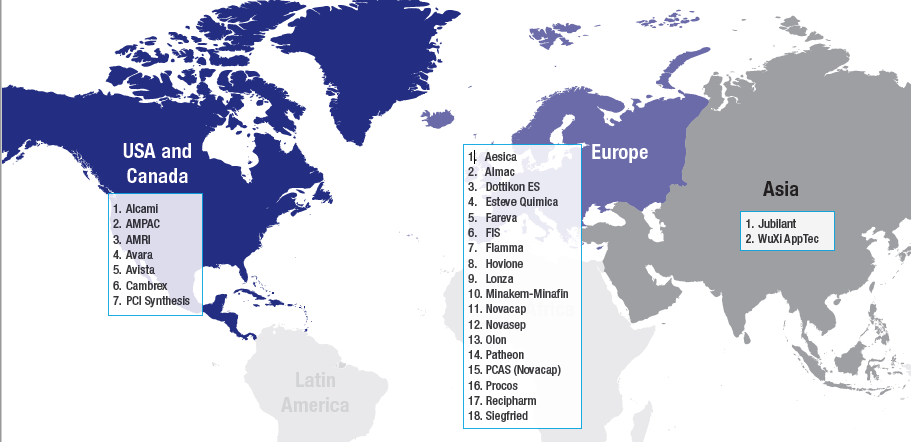
These 27 companies were then compared and contrasted. Using a vast pool of publicly-available information as the data source, 50 unique benchmarking tools were employed to analyse quantitative and qualitative features such as:
- Size and geography
- Company business models
- KPI financials
- Service portfolio and scale capabilities
- Technical capabilities
- Growth, investment and innovation
- Competitive advantage and stress-absorption
- Quality and regulatory.
The findings of these analyses revealed a diverse pattern of business models and capabilities across the globe. The companies included in the study differed in their backgrounds, ownership structures (public, private, private equity owned), sizes (sales from $50 million to $3.8 billion) and business strategies (pure CDMO or with some sales of proprietary products).
Some of them combined drug substance and drug product capabilities, with capacity ranging from early phases to large scale commercial production. A few companies combined drug substance capabilities with expertise in business strategy (pure CDMO or with some sales of proprietary products).
Where’s the money?
The combined total annual turnover of the 25 companies in the study exceeded $12 billion, and Advitech’s study revealed a number of interesting patterns between turnover, profitability, business models and measures of company efficiencies
The combined total annual turnover of the 25 companies in the study exceeded $12 billion, and Advitech’s study revealed a number of interesting patterns between turnover, profitability, business models and measures of company efficiencies
Comparing the different figures from, and features of, the 27 companies, Paolo Magri (the lead author of the study) highlighted opportunities that still exist in the CDMO sector for companies wishing to differentiate themselves. In terms of technologies and capabilities, the goal is simply “to be the best”, independently from the business model, constantly investing in new technologies, offering full service and protecting the business through a diversification strategy. However, it can be easy to miss the fact that many technologies that used to be niche are now essential capabilities.
What else matters?
As an almost obvious conclusion, a strong R&D team is a fundamental asset, adding to a company’s intellectual property (IP) portfolio, which is a basic, rock-solid element of value creation. Companies with strong IP backgrounds are identified and discussed in the report, offering insights into those businesses with an extended life span guaranteed by the corresponding IP protection.
As an almost obvious conclusion, a strong R&D team is a fundamental asset, adding to a company’s intellectual property (IP) portfolio, which is a basic, rock-solid element of value creation. Companies with strong IP backgrounds are identified and discussed in the report, offering insights into those businesses with an extended life span guaranteed by the corresponding IP protection.
Quality issues are probably more common than might be expected; even large companies suffer from quality related problems and regulatory issues. However, quality may be seen as an opportunity rather than a challenge; it can be a very effective differentiating factor. Companies which anticipate regulatory requirements, adapt quickly, and invest in quality, may see immediate returns in terms of customer loyalty.
Conclusion
It’s a big and potentially confusing market, but Advitech’s new study cuts through swathes of data to provide a unique insight into the market value of small molecule APIs produced by CDMOs, and the CDMOs working in this environment. The resulting analyses should be useful for pharmaceutical clients wanting to ascertain which CDMO will bring most added value to an outsourcing partnership, CDMOs wanting to know how they compare with their competitors, and investors needing to know how one target company compares to another. Such intelligence is vital in a competitive world, in which the rise of the CDMOs cannot be ignored.
It’s a big and potentially confusing market, but Advitech’s new study cuts through swathes of data to provide a unique insight into the market value of small molecule APIs produced by CDMOs, and the CDMOs working in this environment. The resulting analyses should be useful for pharmaceutical clients wanting to ascertain which CDMO will bring most added value to an outsourcing partnership, CDMOs wanting to know how they compare with their competitors, and investors needing to know how one target company compares to another. Such intelligence is vital in a competitive world, in which the rise of the CDMOs cannot be ignored.
References
- Principe J. European Pharmaceutical Review, 3rd July 2017.
- ISR Reports. Contract Development and Manufacturing Outsourcing Models, 2016.
About Advitech
Advitech is a Swiss boutique advisory firm focused on the healthcare and fine chemical industries. Combining the classic features of the purest entrepreneurial spirit with best practices of professional organizations, while maintaining the highest standards of integrity, Advitech is able to support its clients in: Corporate Business Development, Management Consulting & Business Intelligence, Products and Technologies Development.



