Cosmetics & Personal Care
A new level of security for pharma and cosmetics supply chains 1st March 2018
By Michael Hogan, PhD, Vice President, Life Sciences at Applied DNA Sciences
Michael Hogan, PhD, Vice President, Life Sciences at Applied DNA Sciences, explains how the advent of DNA-marking technology
.jpg)
Michael Hogan, PhD, Vice President, Life Sciences at Applied DNA Sciences, explains how the advent of DNA-marking technology provides a potential weapon against rampant counterfeiting of pharmaceuticals and cosmetics.
All living things have DNA that identifies them uniquely. For instance, the DNA in a human being can be used by a forensics lab, with complete certainty, to establish identity relative to all other humans. This process works because, at its core, the DNA in any organism acts as a molecular barcode that can be read, much as a conventional barcode can be read by standard technology.
With breakthroughs in nanotechnology, forensic science and material sciences, it has now become possible to formulate DNA into materials that are non-biological: to generate DNA-containing plastics, fabrics, inks, pharmaceutical coatings, oils, emulsifiers and detergents. The advent of this technology is extremely important today as a potential weapon against rampant counterfeiting in two wide-reaching areas: pharmaceuticals and cosmetics.
The pharmaceutical supply chain has been contaminated by counterfeiting and by diversion of legitimate products. Both types of contamination have been magnified by growth in the increase in the number of on-line pharmacies, global and complex supply chains and high margins sought by counterfeiters. The net result has been a loss of brand credibility, a loss of faith in drug regulatory authorities’ review processes, as well as an inability to protect patients in the new electronic marketplace. Perhaps most importantly, the new and greatest risk is the substantial harm incurred by patients who buy “fake” or otherwise substandard medication in these markets. An estimated 1 in 10 medical products circulating in low- and middle-income countries is either substandard or falsified, according to the World Health Organization.1
The newly developed fields of DNA barcoding and DNA-specific formulation chemistry offer a solution. Applied DNA Sciences is currently collaborating with two major pharmaceutical dosage component manufactures in the areas of excipients (inks, film coatings, colourants) and hard-capsule shells. The goal of these collaborations is to expand DNA barcoding to become the new industry standard for control and authentication in the solid oral dosage form-based pharmaceutical supply chain. Similar considerations hold true for the application of DNA barcode technology to cosmetics supply chain control.
How the technology works
DNA barcodes (also known in the pharmaceutical industry as a physical, chemical identifier or “PCID”) are composed of short, unmodified double-stranded DNA molecules generated biochemically. Although synthesized from standard DNA building blocks, the PCIDs have been designed to be a product identifier, only, without any biological function. By fabricating DNA as very small fragments, the DNA-based PCID becomes highly stable with respect to extreme heat, UV light and dryness. DNA barcodes have the capacity to convey a large amount of information. As a result, a DNA-based PCID permits the tracking of a product through the supply chain, employing only femtogram amounts (10-15 grams) of DNA per identifier.
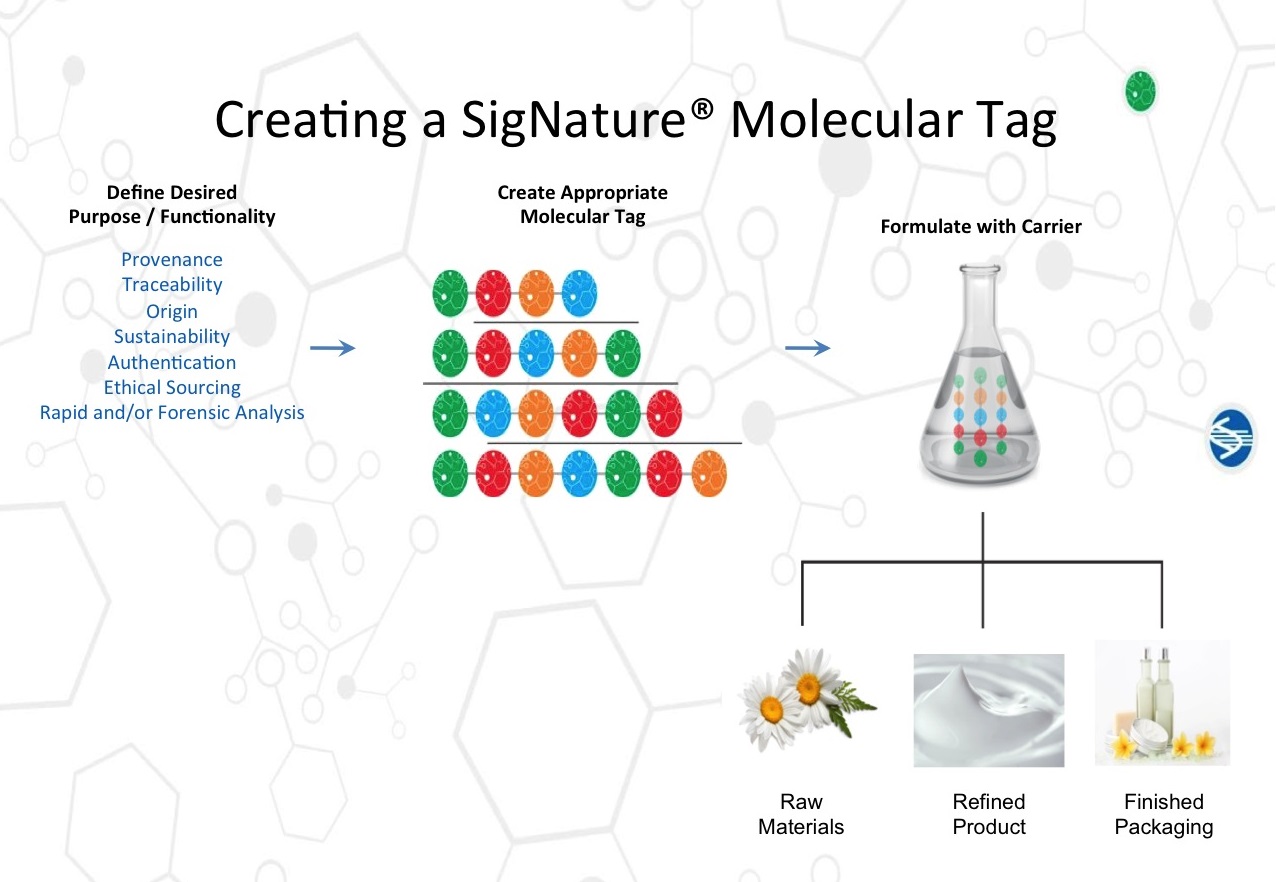
Such industrial-scale DNA formulation is now possible based on the use of two complementary technologies. The first is computer-assisted DNA design and manufacture. It is now possible to assign a bar-code-like message to a small DNA molecule and then fabricate that molecule under computer control so that the resulting DNA barcode can be produced in any amount needed to support its industrial application. The second technology is DNA-specific formulation chemistry. From the perspective of the pharmaceutical and cosmetic supply chain, advanced DNA formulation flexibility means that most pharmaceutical and cosmetic products can now be DNA barcoded. For cosmetics, the DNA barcode can become part of almost any component, including the core formulation. For pharmaceutical tablets and hard-capsule shells, the DNA barcode can become part of the dosage form’s surface coating, API or food-grade ink labelling.
Use in pharmaceuticals and cosmetics
With regard to pharmaceuticals, Applied DNA’s proprietary DNA-based PCID technologies are being combined with the product offerings of two industry partners in the fields of tablet excipients and hard-capsule shells. The collaborations will commercialize platforms for traceability directly on dose, and are intended to significantly reduce the risks associated with counterfeit and falsified medications entering the drug supply chain.
With regard to pharmaceuticals, Applied DNA’s proprietary DNA-based PCID technologies are being combined with the product offerings of two industry partners in the fields of tablet excipients and hard-capsule shells. The collaborations will commercialize platforms for traceability directly on dose, and are intended to significantly reduce the risks associated with counterfeit and falsified medications entering the drug supply chain.
By incorporating DNA-based PCIDs into existing pharmaceutical excipient and hard-shell capsule products, a simple and seamless solution to address counterfeiting and product diversion issues for all solid oral dosage forms is created. The result is the end-to-end protection of a significant portion of the total addressable pharmaceutical market.
The use of DNA-based PCIDs also represents a breakthrough opportunity to help pharmaceutical companies enhance patient safety and reduce risk by using intelligent data and analytics gathered from authentication of the dosage forms themselves. By better understanding trade flows and vulnerabilities in a complex global supply chain, pharmaceutical leaders can be helped to make better decisions on managing distribution patterns, frequent monitoring and deploying preventive measures for their products. When coupled with emerging IT technologies such as blockchain, the power of DNA barcodes only increases.
Similar considerations hold for the ability of DNA barcode technology to address concerns in the cosmetics and personal care sector, where consumers are demanding transparency and truth in labelling, as ethical trade practices are increasingly important issues for worldwide consumers. DNA barcodes can be used to authenticate ingredients such as shea butter and Aloe vera, as well as identify the sources of these ingredients. For example, Applied DNA’s in-house analysis of several commercially available Aloe vera products was unable to detect a conventional molecular marker – known as the “Bar Code of Life” (BCOL) – which may have conclusively revealed the presence of Aloe vera.2 The BCOL may have been damaged during processing of the Aloe vera product. However, further testing showed that when these Aloe vera products were tagged with DNA barcodes, the tags were 100% detectable at extremely minute quantities. 2 This suggests that a wide range of cosmetic products can be forensically tracked and traced with the use of DNA-based PCIDs.
The potential rewards of the application of DNA barcode technology are clear for manufacturers and consumers alike: a world in which we no longer need to worry about the provenance and quality of the pharmaceuticals we depend on to save our lives or the cosmetics and personal care products we use on our bodies.
References
- World Health Organization. Press release, 28th November 2017 (www.who.int/mediacentre/news/).
- Brockway B et al. Internal report, available at http://adnas.com/2017/03/02/signature-molecular-tags-identify-aloe-vera/
Author:
Michael Hogan, PhD, is Vice President at Life Sciences at Applied DNA Sciences, a leading provider of molecular technologies that enable supply chain security, anti-counterfeiting and anti-theft technology, product genotyping and DNA mass production for diagnostics and therapeutics.
E: mike.hogan@adnas.com



