Pharmaceuticals
In full flow – continuously battling bacteria with boron 7th April 2020
By Dr John Studley, Scientific Director, Scientific Update
How can the increasingly large problem of bacterial multi-drug resistance be tackled? Selective inhibito
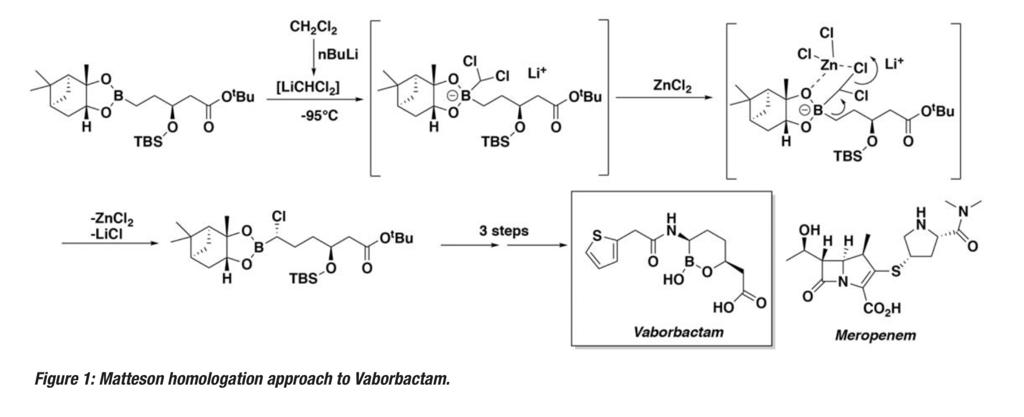
A key step in the synthesis of Vaborbactam is diastereoselective chain homologation of a boronate ester to an a-chloroboronic ester using chemistry originally developed in the 1980s by Donald Matteson (Figure 1).5 This reaction involves generation of dichloromethyl lithium at low temperatures (typically -95° to -100°C), reaction with a boronate ester to generate a boron ‘ate’ complex and subsequent rearrangement to generate the required chain-extended building block. The homologation is carried out in the presence of zinc chloride, which both promotes high levels ofdiastereoselectivity (due to chelation control in the transition state) and suppresses epimerization of the chlorinated product.The requirement for low temperature generation and processing of the organometallic species stems from its instability at higher temperatures, resulting in decomposition via formation of a carbine through a-elimination of LiCl.6 This lithiation-borylation methodology has been further developed by Aggarwal and applied to a number of other complex synthetic targets.7
Scale-up of low-temperature organometallic chemistry in batch is intrinsically challenging for a number of reasons. The ability to remove heat from the reactor during exothermic additions at cryogenic temperatures is difficult and often very high dilutions and slow addition rates are required to maintain control. This in turn can decrease efficiency and productivity. Poor mixing, particularly at the point of addition, can also generate localized hot-spots and variable local stoichiometries resulting in formation of impurities and impacting yield and product quality. Continuous processing is frequently used to mitigate problems of this type encountered during batch processing. Flow chemistry is often the preferred approach in scaling up chemistry that utilizes highly reactive organometallic reagents such as organolithium or organomagnesium derivatives, including unstable species such as the lithium carbenoids utilized in the Matteson reaction.8In situ formation and extremely rapid trapping of the organometallic species before decomposition can occur is possible under flow conditions but is poorly achieved in a large batch reactor.
The Matteson homologation, used to assemble the pivotal Vaborbactam a-chloroboronic ester intermediate (Figure 1), is eminently amenable to a flow chemistry strategy. Based on early pioneering work by a team at Novartis in which millisecond generation and trapping of dichloromethyllithium at higher temperatures under flow conditions was demonstrated,9 Christian Schuster’s team at Patheon (Thermo Fisher Scientific), in collaboration with a wider group of industry experts,successfully utilized this technology, converting a lab-based model reaction into a continuous manufacturing process run on several-hundred-kilogram scale under full cGMP conditions in yields of 97% .10 The corresponding batch process proved unscalable under laboratory conditions. Ultimately several tonnes of Matteson product were prepared in a process validation flow campaign enabling FDA fast-track approval and facilitating rapid development of Vaborbactam.
Key to the success of this work was a detailed investigation into the zinc chloride addition and developing conditions to prevent precipitated solids clogging the system – a mainstay of flow chemistry processes. In early development work, the process flowstream was quenched into a batch vessel containing the pre-cooled zinc Lewis acid.
This quickly became a bottleneck for increasing throughput, however. Two engineering solutions to this problem were explored, a continuous loop quench (mimicking the batch process) and a continuous stirred tank (CSTR)-based cascade quench. In the loop quench, boronate adduct was fed into a circulating pre-cooled solution of zinc chloride with reaction temperature controlled by heat exchangers. The product solution was pumped out and consumed zinc salt was continuously replenished in the loop reactor to keep the molar ratio constant. In the CSTR approach, zinc chloride solution was constantly fed to the pre-cooled vessel, the product solution exiting the CSTR in a cascade system controlled by an overflow device. The loop reactor system was ultimately selected to move forward and enabled the reaction to be run at a higher temperature and with better diastereometric control.
By leveraging the advantages a continuous process can impart, including increased process control, energy efficiency and reduced processing times, Schuster’s team succeeded in taking a difficult, unscalable organometallic reaction to full-scale production. Another victory in the ongoing battle against the bacteria!
References:
-
Evolution of antimicrobial resistance among Enterobacteriaceae (focus on extended spectrum b-lactamases and carbapenamases, J. Lynch et al, Expert Opin. Pharmacother. 2013, 14, 199-210; Three decades of b-lactamase inhibitors, S. Drawz et al, Clin. Microbiol. Rev.2010, 23, 160-201.
-
Meropenem/Vaborbactam: a review in complicated urinary tract infections, S. Dhillon, Drugs 2018, 78, 1259-1270. The first b-lactamase inhibitor approved for clinical use was Clavulanic acid in the 1970s.
-
Discovery of a cyclic boronic acid b-lactamase inhibitor (RPX7009) with utility vs class A carbapenemases, S. Hecker et al, J. Med. Chem. 2015, 58, 3682-3692; T. Eisenman, FDA approves new antibacterial drug, August 29th2017.
-
Synthesis of biologically active boron-containing compounds, H. Zhou et al, Med. Chem. Commun. 2018, 9, 201-211; The versatility of boron in biological target engagement, A. Yudin et al, Nature Chem. 2017, 9, 731-742. The first boron-containing drug, Bortezomib (Velacade), was approved in 2003.
-
Homologation of boronic esters to a-chloro boronic esters, D. Matteson et al, Organometallics, 1983, 2, 1529-1535; a-halo boronic esters: intermediates for stereodirected synthesis, D. Matteson Chem. Rev. 1989, 89, 1535-1551.
-
Stability and reactivity control of carbenoids: recent advances and perspectives, V. Gessner, Chem. Commun. 2016, 52, 12011-12023.
-
Lithiation-borylation methodology and its application in synthesis, V. Aggarwal et al, Acc. Chem. Res. 2014, 47, 3174-3183.
-
Flow technology for the genesis and use of (highly) reactive organometallic reagents, R. Luisi et al, Chem. Eur. J. 2020, 26, 19-32; A perspective on continuous flow chemistry in the pharmaceutical industry, M. Baumann, M. Smith et al, Org. Process Res. Dev. 2020, ASAP 10.1021/acs.oprd.9b00524.
-
Dichloromethyllithium: synthesis and application in continuous flow mode, J. Sedelmeier et al, Org. Lett. 2017, 19, 786-789.
-
Development of a continuous flow process for a Matteson reaction: from lab to full-scale production of a pharmaceutical intermediate, C. Schuster et al, Org. Process Res. Dev.2019, 23, 1069-1077.


