Pharmaceuticals
Process intensification with flow chemistry and commercial scale-up 17th June 2021
Dr Stephen D. Drake, Director of Marketing and Development at Kaneka Americas Holdings, Inc describes how commercial processes can be developed through process intensification using enzymatic transformations and ow chemistry, particularly to support difficult small-molecule route development, scale-up and manufacturing.
Pharmaceutical and CRO/ CMO companies are continuously looking for ways to improve chemistry and manufacturing processes. The goal, of course, being to make pharmaceutical API and intermediates in the fastest, cheapest, and most efficient way possible. Novel/innovative technologies and ideas are being put into practice for new, existing, and old (sometimes ancient) manufacturing processes and this approach has developed into an idea called ‘process intensification.’
Process intensification focuses on the development of novel technology, equipment, and techniques that are expected to bring dramatic improvements in manufacturing and processing productivity and efficiency. These improvements, whether applied to development or existing manufacturing programmes, provide value in many ways, cost savings, process safety, decreases in equipment size/ production-capacity ratios, energy consumption, waste production, and atomic efficiency, ultimately resulting in cheaper,more sustainable eco-friendly manufacturing processes.
The advantages of process intensification have already been realized in industries outside chemical and pharmaceutical manufacturing. The application of these existing technologies to pharma is a great starting point, and potentially low hanging fruit. At Kaneka, the polymers division has been using continuous manufacturing, also called ow chemistry, to develop and scale manufacturing processes for customers for decades, and this knowledge was utilized to develop the company’s continuous manufacturing and development technologies for the pharmaceutical API and intermediates market.
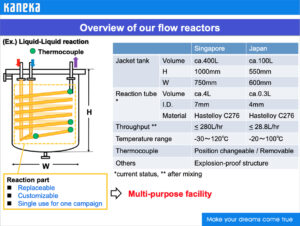
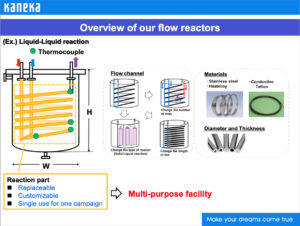
Flow reactors in use at Kaneka’s manufacturing facilities.
Continuous manufacturing process intensification:
Continuous manufacturing is an excellent example of process intensification. The commonly used practice of batch chemistry is still the industry standard and provides its own benefits, of course, since virtually all CMOs have this capability, and the history of proven reliability has been largely explored and is well understood. This approach to manufacturing, however, does not always lead to the most efficient or cost effective, sustainable processes that can be used at commercial scale.
In traditional batch chemistry, reagents are added to a stirred tank and the desired product is formed using specified reaction conditions such as temperature, mixing times, reagent addition times, etc. Any reaction issues or mistakes that affect yield, product quality or by-product profile may result in a failed or partially unusable batch, not only delaying material supply but also costing significant money in lost product, and large amounts of potential waste if the failed batch cannot be reworked.
With a continuous manufacturing process developed based on process intensification principles, starting materials, solvents, and reagents needed
to form the product are sent through a reaction tube, having inherently less volume, where a heating/cooling jacket adjusts the temperature as needed while the reaction components are simultaneously added. Chemists can then determine in real time the reaction parameters that affect product quality and yield and can adjust these parameters in real time to deliver the product within the targeted specifications. Because a ow process can be used to precisely tune the reaction conditions variably, product yield and reaction efficiency can be increased and respond to any irregularities or anomalies. As a result, continuous manufacturing can consistently deliver high product quality from small volumes up through large commercial volumes over the various phases of the clinical development cycle of a project.
The early development approach to this chemistry takes some additional training from the standard lab practices mentioned earlier, as well as the inclusion of some chemical engineers into the manufacturing team, but this investment allows a variety of difficult chemistry, including high-energy reactions, dangerous reactions (exothermic or toxic), control over kinetic vs thermodynamic products, and a myriad of other chemistries to be explored and developed in both academic and industrial research labs.
Investments at Kaneka
Kaneka has invested in continuous- ow technology to expand its existing expertise using enzymatic transformations for small molecules, as well as developing new chemistries including phosgenation. The company’s ability to perform phosgenation under continuous- ow conditions at commercial scale gives access to a powerful, yet toxic and dangerous reaction for both discovery and manufacturing programmes, under safe and reproducible conditions.
There are many CRO/CMOs and pharmaceutical companies investing in this technology at laboratory scale, and if and when scale-up is needed, having the right partner or equipment is essential. Kaneka has designed its pilot – and commercial – scale continuous reactor system to closely mimic its laboratory equipment to allow for quick and easy scale-up, with a typical lead time of 5-6 months to go from a gram sample to tonne capabilities as seamlessly as possible, and to a global customer base.
The FDA has provided easy access and encouragement to the pharmaceutical industry in applying continuous manufacturing to existing or new manufacturing processes. Working with an experienced CDMO like Kaneka can be an easy, and valuable entrance into applying continuous- ow, and other Process Intensification technologies to small molecule drug programmes.

Reactor capacities at Kaneka’s manufacturing subsidiary Osaka Synthetic Chemical Laboratories, Inc.

Reactor capacities at Kaneka’s manufacturing subsidiary Kaneka Singapore Co. (Pte) Ltd.
Meet Stephen Drake of Kaneka
Stephen Drake received his PhD in Organic Chemistry from Georgetown University in 2011 under Professor Travis Holman in the eld of supramolecular chemistry. In 2011 he joined Johnson Matthey as a Business Development Manager for the catalysis group, specializing in metal-mediated and enzymatic catalysis. He joined Kaneka in 2016 and is now the Director of Marketing and Development for North America, focusing on process intensi cation using enzymatic transformations and ow chemistry. Currently he is responsible for R&D and commercial collaborations for North American companies using these technologies to support dif cult small-molecule route development, scale-up and manufacturing.


