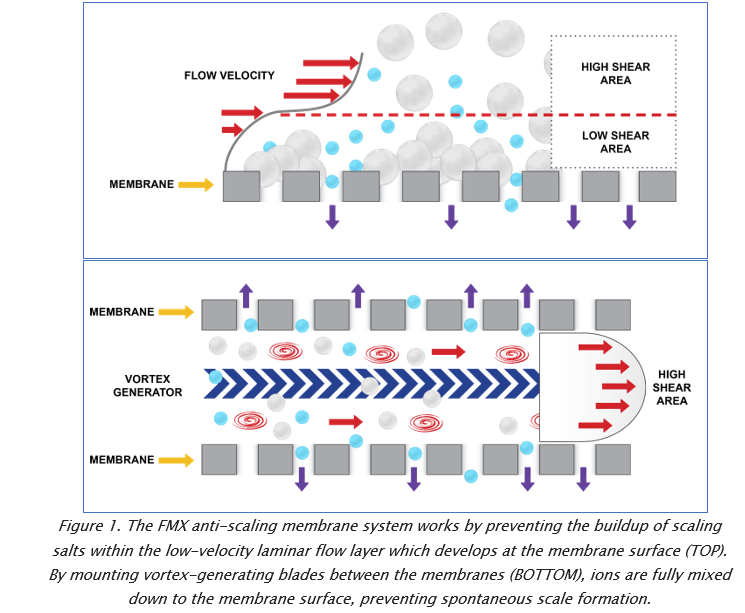
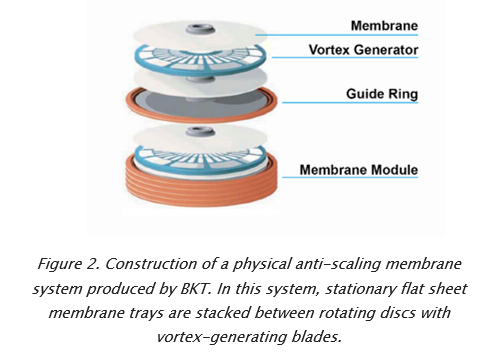
Jon Liberzon, Vice President of BKT Co, discusses the challenges of removing toxic compounds from flue gas desulfurization (FDG) wastewater, and considers the best available technologies to facilitate compliance with effluent regulations.
To upend the old adage, where there’s fire, there’s smoke. Burning fossil-fuels in power plants produces exhaust gas, which contains elements, including sulfur, that originated in the combusted fuel. Flue gas from coal plants, incinerators and some other facilities must be scrubbed free of sulfur dioxide (SO
2) in order to comply with clean air regulations.
Usually, this means installing flue gas desulfurization (FGD) scrubbers, which use sorbents to capture and convert the SO2 released during combustion into bound or dissolved sulfates. In wet scrubbers (the most common type), flue gasses contact a lime slurry which absorbs sulfur and precipitates gypsum, leaving behind a wastewater rich in sulfates. This wastewater must be treated before it is discharged to the environment.
Unfortunately, during combustion, fossil fuels release more than just SO2. Flue gasses contain a veritable cocktail of elements, including heavy metals, that are absorbed in FGD scrubbers and end up in wastewater. Removing these elements represents a major expense for power-plants, which have been known to shut down in response to new discharge regulations. In 2015, the EPA tightened limits on four of these compounds: mercury, arsenic, selenium and nitrate/nitrite. The new, stricter rules were introduced as amendments to the Effluent Limitation Guidelines (ELG), a series of technology-based requirements first established in 1974 and updated five times in four decades. These guidelines establish physical/chemical and biological treatment as the best available technologies to remove these four target compounds from FGD wastewater, but that designation may not last long.
Business as usual: best available technologies
Physical/chemical and biological treatment systems target specific compounds. Biological treatment systems, for example, use bacteria to convert dissolved selenate and nitrates to nitrogen gas and elemental selenium. Physical/chemical systems generally focus on mercury and arsenic.
To manage all four target compounds, multiple treatment processes are required, and the remaining wastewater still contains other constituents which may be regulated at the state or local level, such as chlorides, boron, bromides and other salts. This means that effluent from these systems is not suitable for industrial reuse, and may not even be dischargeable in some cases. For this reason, some power plants have opted for zero-liquid-discharge (ZLD) systems which evaporate the liquid water, leaving the dissolved salts as concentrated solids, which are more easily disposed of. Despite producing pure water that can be reused in cooling towers or other parts of the plant, evaporation remains an incredibly expensive solution for FGD wastewater streams that can run into millions of gallons per day.
Deja-Vu for thermal evaporation?
In the mid-fifties, when scientists began commercializing desalination processes for drinking water from the sea, they used evaporation to separate salt from water. These plants were effective at producing pure distilled water, but they were energy hogs. Evaporative desalination systems have long since been superseded by a new, more energy efficient technology for separation of dissolved solids from water: membranes.
Membrane technology has made huge leaps in the last decade, with prices falling much faster than other treatment technologies. Adoption of reverse osmosis (RO) membranes, which remove all salts from water, has exploded in a range of sectors, from seawater desalination to ultrapure water production, industrial treatment and municipal wastewater reuse.
One sector where this technology has been slow to advance, however, is in FGD treatment. The reason? High concentrations of divalent ions such as sulfates, silicates, calcium, magnesium and barium form hard scales on membrane surfaces during filtration. These sharp or sticky crystals plug up pores and perforate the membrane, greatly reducing its throughput and lifespan. In some cases, scale can rapidly and permanently damage entire membrane trains, forcing budget-busting replacements of entire filtration systems.