Oilfield & Energies
Chemical corrosion control in the oil and gas sector 20th May 2019
By Henry Craddock, Director of HC Oilfield and Chemical Consulting
Corrosion and its control are highly important subjects in modern engineering and construction. This is a key discipline in the oi
Corrosion and its control are highly important subjects in modern engineering and construction. This is a key discipline in the oil and gas sector, particularly in the offshore environment where usual inspection techniques cannot be employed, says Henry Craddock, Director of HC Oilfield and Chemical Consulting.

Corrosion control seeks to minimize the costs associated with corrosion and its potential failure. The selection of materials used in any given engineering scenario is subject to a number of risk and failure criteria around possible corrosion. This is especially the case in the upstream oil and gas sector and associated pipeline transport system, an area where in 2001 a US government report estimated the cost of corrosion at $1362 billion per annum.1 It is against this economic imperative that chemical inhibitors are assessed for their applicability in the protection and mitigation strategies for the retardation of corrosion within the oil and gas process industry.2
A number of chemistries have been successfully applied to corrosion problems encountered in a number of industries: amines, quaternary salts of amines and imidazolines.3 All of these chemistries act as filming protection agents, either by filming on the metal surface or with a variety of associated scales (e.g. calcium carbonate deposited on the surface). In addition to filming characteristics, it is essential that corrosion inhibitors exhibit properties to slow the corrosion rate. This is usually through a combination of increasing the anodic or cathodic polarization behavior, thereby reducing the movement or diffusion of ions to the metal surface, and increasing the electrical resistance of the metallic surface.
Filming protection is the most widely used method of corrosion inhibition in oil and gas processing and transportation by pipeline. This is due to the necessity to have good protection against corrosion within a highly dynamic environment. The mixture of crude oil, dissolved gases, condensate, connate and other waters (brines) can give rise to a highly aggressive and corrosive environment. An additional challenge is that these mixtures are often under high flow, creating a number of shear stress conditions.
Traditionally, chemical corrosion inhibitors have been sought which have the ability to film within fluid mixtures at the surface between liquid and solid (i.e. surfactant-like materials),4 and are also persistent under conditions of high flow (i.e. have a certain amount of persistence to removal within these conditions).5 These materials are, in general, excellent corrosion inhibitors under a variety of field conditions. However, they possess certain boundaries to performance, such as conditions of high temperature, and have certain properties relating to marine toxicity, bioaccumulation and biodegradation which make them less acceptable for use in highly regulated offshore environments in the North Sea and North East Atlantic (those areas governed by the OSPAR Convention).
There is a dilemma between producing more environmentally friendly inhibitors and compromising the technical effects that you are trying to design. For example, persistent materials are not usually readily biodegradable. Similarly, a number of the chemistries are nitrogen based and possess inherent toxic properties.
It is in these areas of extreme conditions and environmental acceptability that chemistries other than the traditional surfactant-based materials continue to be explored. However, in the area of environmental acceptability, maintaining desired surfactant properties and persistency leads to compromise against acceptable environmental criteria in terms of biodegradation toxicity and persistency.
A good example of the environmental dilemma against the cost imperative is the use of polyaspartates based on the amino acid aspartic acid. These products have reasonable corrosion inhibitor properties and are highly biodegradable, however, they require much higher dose rates that traditional amines or quaternary ammonium salts.6
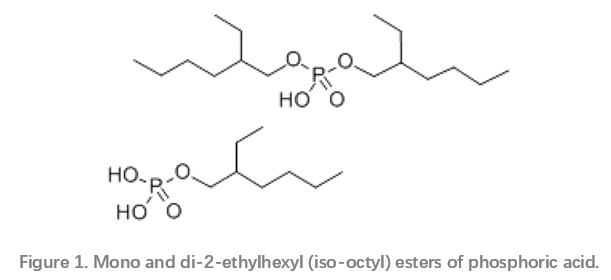
A key group of environmentally acceptable products are the phosphate esters. The ethoxylated phosphate esters in particular are used in different areas of corrosion inhibition in the oil and gas sector, such as drilling fluids, well stimulation, oil and gas production and pipeline transportation. They are very effective, especially at moderate temperatures or in the presence of trace amounts of oxygen.7–9 Mono- and di-phosphate esters (Figure 1) have also been found to be useful in mitigation of under-deposit corrosion.10 The phosphate esters containing a hydrophobic nonyl phenol group have been shown to be better film forming corrosion inhibitors (FFCIs) than linear or branched aliphatic phosphate esters, with the di-esters being more effective than the monoester.
Phosporous compounds are, however, under scrutiny by regulators due to their potential eutrophication effects.
In high temperature applications thermal stability and persistency of film are critical in maintaining performance. However, these are properties that often compromise environmental acceptability as rates of biodegradation are often low and marine or aqueous toxicity can be high. Finding chemical inhibitors which are environmentally acceptable and offer performance at temperatures over 140oC remains a significant challenge in oilfield chemistry.
References:
- Ruscau G, Al-Anezi A. Oil and Gas Exploration and Production. Report FHWA-RD-01-156, September 2001.
- Webster S et al. CORROSION-93 Conference, Houston, Texas, USA, 1993; Paper No. 109.
- Raman A, Labine P (eds). Reviews on Corrosion Inhibitor Science and Technology 1993; Volume 1.
- Craddock H. Oilfield Chemistry and Its Environmental Impact. John Wiley & Sons, Oxford, UK, 1993;Chapter 3.
- De Marco R et al. Corrosion 2002;58: 354–63.
- Kalota DJ, Silverman DC. Corrosion 1994;50: 138–45.
- Dougherty JA, CORROSION-98 Conference, San Diego, California, USA, 1998; Paper No 98015.
- Alink BA et al. CORROSION 99 Conference, San Antonio, Texas, USA, 1999.
- Yepez O et al. SPE International Symposium on Oilfield Chemistry, The Woodlands, Texas, 2015; Paper No. 173723.
- Brown B et al. Corrosion 2015;71:1500–10.
Author:
Henry Craddock, Director of HC Oilfield and Chemical Consulting, Kirriemuir, Angus, Scotland
T: +44 (0) 1575 572304


